The future of electric mobility is linked to the development of solid state batteries which will certainly be able to give a decisive boost to the spread of electric cars. The advantage over traditional lithium battery (with liquid electrolyte) it's thesignificant increase in autonomy and reduction of charging times.
Solid state battery
At the center of the revolution is the electrolyte: le solid state batteries they are an evolution of lithium ion batteries where the electrolyte is no longer liquid, but solid, but not only that.
In a traditional lithium battery there are 4 elements: anode (-), cathode (+), liquid electrolyte and a separator. During the load, lithium ions flow from the anode to the cathode (anode → canode) moving in the liquid electrolyte passing through the separator. During the discharge the process is reversed, the ions pass from the cathode towards the anode (cathode → anode).
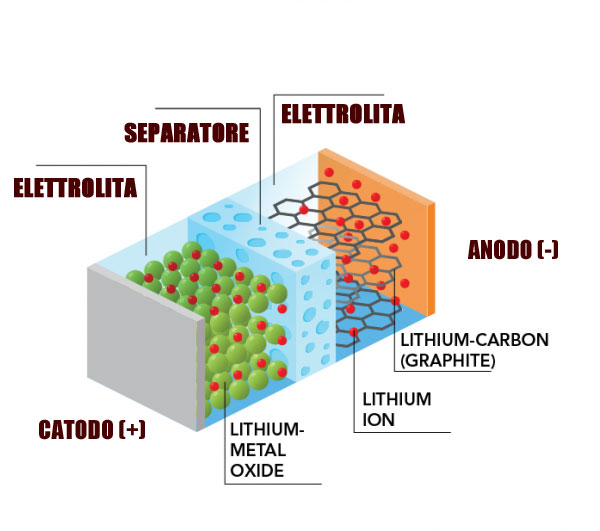
In solid state batteries the elements are reduced to three, with anode, cathode and electrolyte which becomes solid with a dual function ionic conductor and separator and the task of transmitting electrical energy fromnegative electrode (anode) to that positive (cathode) and vice versa.

L'electrolyte solid can be made with different materials and be polymeric, ceramic or other types.
- Polymeric materials: Solid polymer electrolytes are made of ionic conductive polymers, which allow the transport of ions through their molecular structure. These polymers can be synthesized in the laboratory or obtained from natural sources. Examples include lithium salt-modified polyethylene oxide (PEO), poly(ethylene oxide)-poly(dimethylsiloxane) (PEO-PDMS), poly(ethylene oxide)-poly(propylene oxide) (PEO-PPO), and others. Polymer electrolytes offer mechanical flexibility and can be processed into thin films, allowing integration into different cell geometries.
- Ceramic materials: Solid ceramic electrolytes are composed of crystalline or glassy structures that facilitate ionic transport. These ceramic materials include oxides, phosphates, sulfides, and mixed-state solids. Some common examples are lithium aluminum oxide (LiAlO2), lithium titanium oxide (Li4Ti5O12), lithium sulfur sulfide (Li2S-Li2S2), and others. Ceramic electrolytes are known for their high ionic conductivity at room temperatures and their chemical stability.
- Hybrid materials: Some solid-state batteries use hybrid solid electrolytes, which combine polymer and ceramic material properties. These materials can offer better ionic conductivity than pure polymers and greater flexibility than pure ceramics. For example, polymer-ceramic electrolytes combine ionic conducting polymers with ceramic nanoparticles to improve overall battery performance.
In a solid state battery, the liquid electrolyte present in lithium ion batteries (see image below is the battery on the left) is then replaced bysolid electrolyte (battery in the image below on the right) in less overall volume so that, in addition to providing a higher energy density Wh/m3, occupy less space with equal capacity.
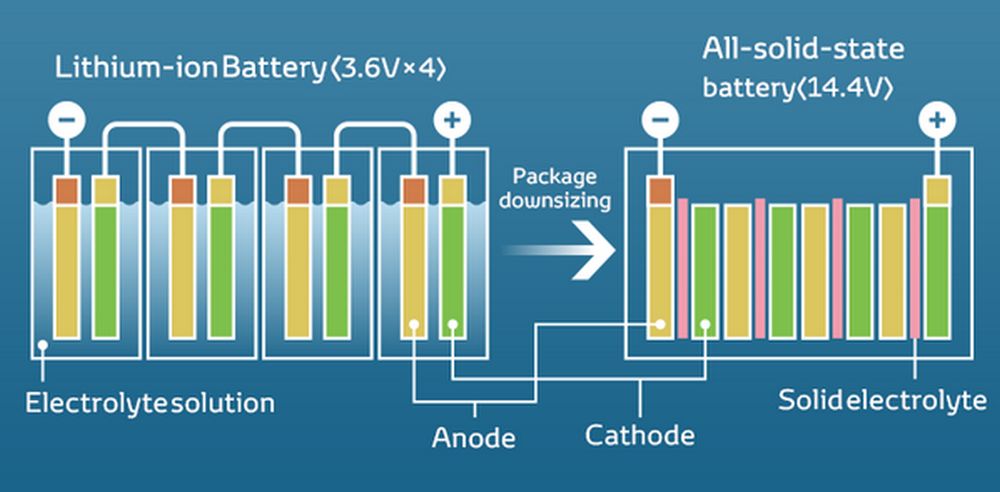
On a geometric-dimensional level, in the image above you can also see the smaller volume occupied by a solid state battery with the significant difference in the distance between anode and cathode, much greater in traditional lithium batteries. It is obvious that the variation in volume of the electrolyte between liquid and solid depends mainly on the structure and density of the materials used. However, we can consider some general factors that influence this change:
- The Volume of the liquid electrolyte it is usually larger and almost always takes up more space than solid electrolytes. This is because the liquids distribute and adapt to the shape of the container in which they are inserted, filling it completely. The volume occupied by the liquid electrolyte depends on the amount of liquid needed to completely immerse the electrodes and allow the transport of the ions. And you cannot go below certain values. In this case we must also consider the additional volume of the “separator”.
- The Volume of the solid electrolyte it is generally lower than that occupied by liquid electrolytes. This is because solid materials have a more compact structure and occupy less volume compared to their liquid equivalent. However, it is important to consider that even solid electrolytes can have some porosity or be compressed to a certain extent, which can affect their total volume.
By reducing the volume necessary for the solid electrolyte which also incorporates the function of separator, there is consequently a greater overall energy density of the battery.
CONS In terms of specific volume changes, it depends on the specific characteristics of the materials used and the operating conditions of the battery. For example, some solid electrolytes can expand or contract during the battery charging/discharging process, which can affect the overall volume of the system.
Greater instantaneous current delivery capacity
In a solid state battery the capacity of individual cells to deliver current is up to 5 times higher: the coin-sized cells offer a double energy density and could therefore, with the same battery volume, almost double the autonomy with a single charge.
This feature obviously also reflects on charging times which are significantly shorter.
They are safer
LSolid-state batteries are also safer as solid electrolytes significantly reduce the risk of fire or explosion, which can occur with traditional batteries due to electrolyte leaks or short circuits. This makes solid-state batteries a safer choice, especially in applications where safety is a priority, such as in electric vehicles.
They last longer
Solid state batteries tend to have a longer lifespan than traditional batteries, thanks to greater stability of solid electrolytes. This means they can withstand a greater number of charge/discharge cycles without suffering significant performance degradation.
We also consider how the SOH (State of Health) varies over time and by number of charging cycles that of a traditional lithium battery compared to a solid state battery.
Let's see how the SOH degradation changes over time for a blithium battery with liquid electrolyte:
- SOH over time: Over time, traditional lithium batteries tend to experience a gradual decline in performance, primarily due to processes such as dendrite growth and the formation of passivating films on the electrodes. These phenomena can cause an increase in the internal impedance of the battery and a decrease in its overall capacity, thus reducing the SOH over time.
- SOH by number of cycles: Traditional lithium batteries show a gradual decline in performance as they are subjected to charge/discharge cycles. This is due to degradation of the electrodes and electrolyte during cycling, which can lead to a decrease in battery capacity and efficiency. The SOH of a traditional lithium battery can decrease significantly after a relatively large number of cycles, usually on the order of hundreds to a few thousand cycles, depending on usage conditions and battery design.
Solid State Battery:
- SOH over time: Solid-state batteries tend to have greater stability over time than traditional lithium batteries. Solid electrolytes reduce the risk of phenomena such as dendrite formation and electrode degradation, helping to maintain a higher SOH over time. However, some challenges such as the growth of solid interfaces and the formation of interfacial films can still affect SOH over time, albeit to a lesser extent than with traditional batteries.
- SOH by number of cycles: Solid-state batteries tend to have greater resistance to degradation during charge/discharge cycles than traditional lithium batteries. Solid electrolytes offer greater stability and less tendency for deposits or films to form on electrode surfaces, which can help maintain higher SOH even after a high number of charging cycles. Solid-state batteries are expected to be able to withstand tens of thousands of charge/discharge cycles with a relatively high SOH, although research and development in this area is still ongoing to further optimize performance.
In summary, solid-state batteries tend to maintain a higher SOH over time and during a greater number of charging cycles than traditional lithium batteries. This makes them a very attractive choice for applications that require long-term high performance and longer battery life.
On the other hand, they have a higher production cost which is currently almost double compared to a traditional lithium battery with liquid electrolyte.
With the same dimensions, the solid-state battery ensures electric carsautonomy increased with a reduction in the gap compared to that of cars with petrol and diesel engines, also allowing for the reduction of charging times with waiting times at the charging stations becoming significantly shorter.
You might be interested in them (in fact I recommend them)

→ Selected and tested electric cars to buy
→ Electric car prices and features
→ CALCULATE ELECTRIC CAR CHARGING TIME
→ Electric car charging cost
→ Video tests of ELECTRIC CARS
Testing new electric cars
→ EV Driving all about electric and hybrid cars
→ What do you think? Drop by discussions on the FORUM!
The article Solid state battery, leap in quality thanks to the solid electrolyte comes from newsauto.it.
#Solid #state #battery #leap #quality #solid #electrolyte