OfRuggiero Corcella
Gavi Alliance and Unicef announce that the Euvichol-S vaccine has received prequalification from the World Health Organization. The green light will allow production to be increased. Meanwhile, the distribution of 1.2 million rapid diagnostic test kits is underway in 14 countries
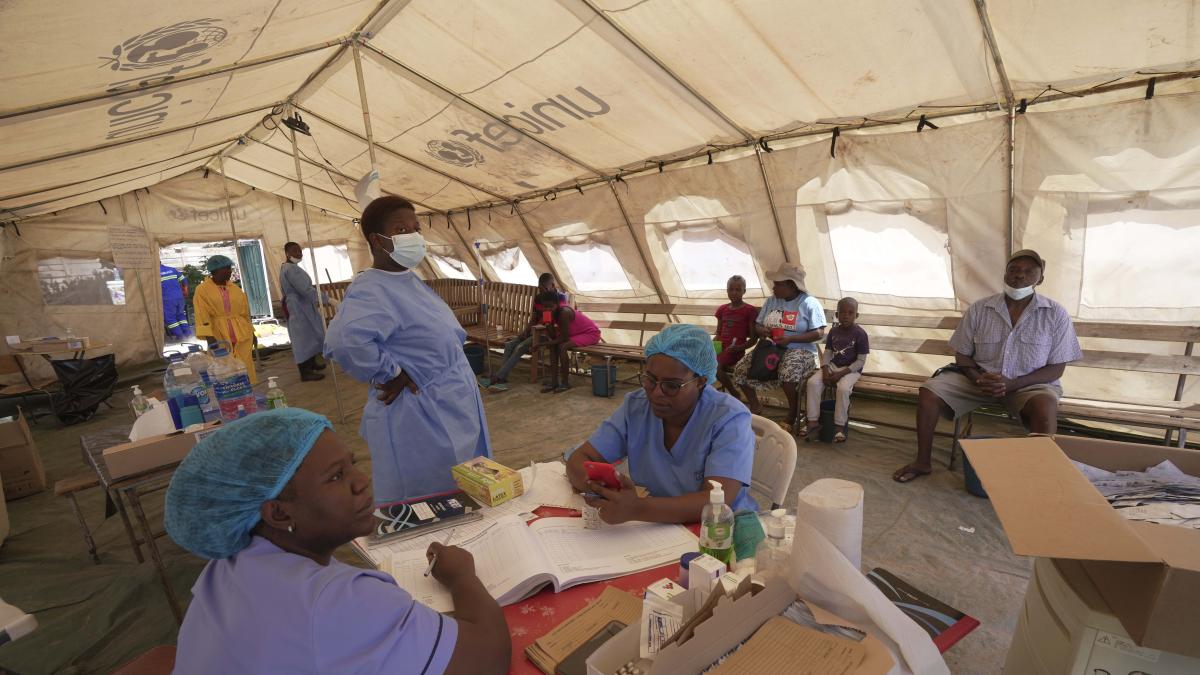
Cholera continues to be scary. Especially in Africa. But now the situation is becoming dramatic in different parts of the world. To try to respond to the emergency, we are moving on two fronts: early diagnosis and vaccines.
Rapid diagnostic test kit
Rapid diagnostic test (RDT) kits for cholera arrived in Malawi on April 5, marking the start of a global program to distribute more than 1.2 million tests to 14 high-risk cholera countries in over the next few months. The global cholera diagnostic program is funded and coordinated by Gavi, the Vaccine Alliance (Gavi), with sourcing and delivery to countries led by Unicefand undertaken in collaboration with the Global Cholera Control Task Force (GTFCC) and theWorld Health Organization. It was developed in collaboration with Findwho led the development of a target product profile describing the required characteristics of RDTs for cholera, and with other organizations.
Now, as Gavi and Unicef report, a new oral vaccine against cholera (OCV), Euvichol-S, has received WHO prequalification and can be made available to countries around the world. The prequalification of this new product will help EuBiologics, the manufacturer, to develop larger volumes of vaccine, more quickly and at lower costs, representing a key step in expanding supply in response to the current rapid global increase in cholera epidemics.
Today's approval will help increase the overall supply of oral cholera vaccines (OCV) available in 2024. It is expected that approximately 50 million doses will now be available in global storage this yearup from 38 million in 2023. Euvichol-S is a major product innovation: a simplified formulation of Euvichol-Plus that reduces the number of vaccine components – providing a vaccine that studies have shown remains equally effective against the main cholera serogroups while reducing the cost and complexity of production – thus allowing larger volumes to be produced faster.
In 2023, there will be 700 thousand cases of cholera in the world
Cholera has increased globally since 2021,with 473 thousand cases reported to WHO in 2022, more than double those reported in the previous year 2021. Preliminary data for 2023 reveals further increases, with over 700,000 cases reported. Many epidemics have high mortality ratesabove the 1% threshold used as an indicator for the early and adequate treatment of cholera patients.
This rise in numbers is even more dramatic when you consider that Cholera is a preventable and treatable disease and that cases had been declining in previous years.
The large number of epidemics led to one unprecedented demand for vaccines from affected countries. Although the global supply of OCV increased eighteen-fold between 2013 and 2023, the large and steady increase in demand has put pressure on global OCV storage. Partners and countries are working urgently on cholera response, prevention and control measures in the face of this crisis, and have called on countries, producers and others to provide support.
Approval arrived at an important moment
«The prequalification of Euvichol-S represents a lifeline for vulnerable communities around the world,” says Derrick Sim, general manager of Vaccine Marketplace and Health Security at Gavi. «Every dose of vaccine delivered through Gavi programs today represents years of planning and investments to shape the market so that the offering matches the needs of the countries. The approval of this new product could not have come at a more important time, given the acute increase in cholera epidemics we are seeing around the world. We commend EuBiologics for their role in ensuring that countries around the world have access to the cholera vaccine as part of their response kit.”
At the moment, EuBiologics is the only OCV supplier to the global repository, although that is expected other manufacturers have products available in the next few years. In March, ICG members appealed to governments, donors, vaccine manufacturers, partners and communities unite in an urgent effort to stop and reverse the rise of cholera
The role of Gavi and Unicef
Gavi works to shape the OCV market and finances the global storage of OCV doses, along with transportation and vaccination activities in low-income countries. Unicef responsible for purchasing and delivering doses to countries. The use of the depot for emergency response is overseen by the International Vaccine Supply Coordination Group (ICG), led by WHO.
“Despite cholera being preventable and easily treatable, children continue to suffer from this potentially lethal disease. Unicef has already guaranteed access to all available doses of the newly approved vaccine and will deliver them to the countries to the highest possible extent”, underlines Leila Pakkala, director of the Unicef Supply Division.
«Approval means that UNICEF can increase procurement and delivery of cholera vaccines by more than 25%combating deadly cholera epidemics more forcefully.”
#Cholera #green #light #oral #vaccine #million #doses #year